Enhancing digitization, using quality and manufacturing platforms, and integrating technologies will open the door to a world of previously hidden data—data that companies can leverage to drive innovation.
Principle 3: Agile and collaborative teams
Technology integrations also allow real-time visibility into operations and processes across departments. This visibility can significantly enhance quality control, make it easier to identify bottlenecks, reduce errors, and ultimately improve product quality and time to market.
Conclusion
Once products are in the market, it’s critical to create customer feedback loops. Gathering data from customers can help an organization understand product performance and customer satisfaction as well as identify defects or inferior product. Even with the greatest care, recalls still happen. In fact, during the first quarter of 2023, medical device recalls increased 4.6% to 252 events over the previous quarter, and pharmaceutical recalls were the highest in a single quarter in the past 18 years, at 144 events. Organizations that have a consistent method for collecting data from customers, prioritizing customer needs, and aligning that information with their quality processes will experience improved customer satisfaction, loyalty, and positive brand sentiment. Furthermore, using customer feedback to drive development of next-generation products is a surefire way to improve business outcomes.
The purpose of a Quality 4.0 initiative is to enhance connectivity and the culture of quality throughout the enterprise; therefore, the organization must have a digital transformation strategy. According to MasterControl’s industry research, more than half of respondents place digital maturity as a high or the highest priority for the organization. Understanding that achieving Quality 4.0 isn’t an overnight strategy, companies can now align technologies across the organization and look for ways to synergize across departments.
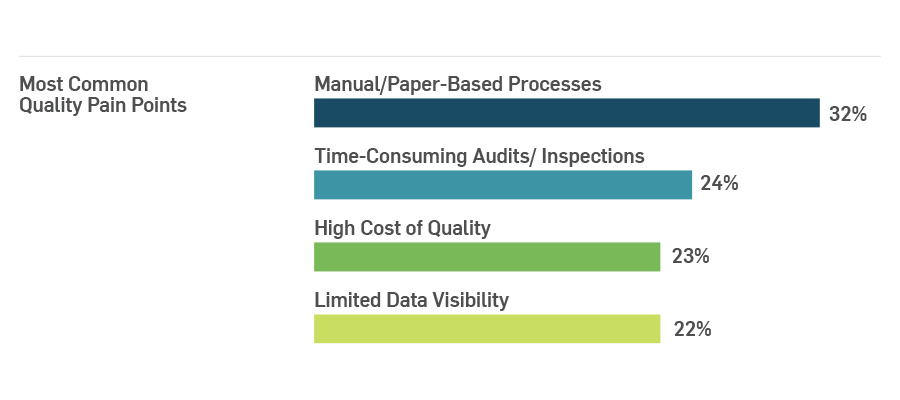
The technology advancements available today can lead to improved quality processes and more efficient operations. Yet we still see paper processes lingering in many life sciences organizations. MasterControl recently conducted a survey to assess the digital maturity of life sciences organizations, and 32% of respondents stated that manual, paper-based processes are still a very significant pain point in their operations. Smarter, more connected technologies are revolutionizing the way medical devices and pharmaceutical products are developed, manufactured, and used.
Keep in mind, Quality 4.0 doesn’t happen through one department alone. It takes a village. From leadership down to the day-to-day implementation, quality must be embedded in the organization’s culture to be effective and create long-term value. There are four foundational principles that every leader should evaluate as they build a culture of quality centered around digital transformation and Quality 4.0 initiatives.
Principle 1: Customer-centricity
More data pass through quality and manufacturing than any other department. Companies that don’t harness the data from that rich source of information will not be able to optimize their processes or identify issues before they arise. Leveraging data analytics and artificial intelligence (AI) can help companies take large amounts of quality and manufacturing data and make sense of them faster than a human ever could. But for that to happen, those data must be digitized. Siloed systems mixed with paper processes lead to disjointed data and create more work for quality professionals as they try to bring information together and create a single source of truth.
Let’s start with a definition of Industry 4.0, keeping in mind that we’re rapidly approaching Industry 5.0. Industry 4.0 is an era marked by enhanced digitization and the increased connectivity of smart technologies. Where Industry 5.0 is more values-driven, it will require the technology of Industry 4.0 to achieve full potential. Quality management plays a vital role for both.
This principle is a reminder that customers and their satisfaction are core drivers for any business. Maintaining that awareness in quality initiatives is imperative to overall company success.
Principle 2: Data-driven decision making
Providing high-quality, effective products is a must for any life sciences company. Inherently, these products are meant to provide treatments, reduce suffering, and save lives, so it’s only natural that they must be developed and manufactured to be safe and effective. But outside of that, delivering them to patients as quickly as possible, without compromising quality, should also be a driver.
References
MasterControl. “The State of Digital Quality Maturity in Pharma and Medtech.” Research report.
Sedgwick. “U.S. Product Recalls Hit Four-Year High in First Quarter of 2023.” PR Newswire, May 25, 2023.